🔹 Free EMC consultation: Optimize designs for compliance and cost
🔹 Global material database: Multi shielding materials for all budgets
🔹 Rapid prototyping: First samples in 7 days, low-MOQ support
In the design and manufacturing of medical devices, electromagnetic interference (EMI) is a critical concern. Even minor interference can lead to data inaccuracies, false alarms, or even misdiagnosis. EMI shielding enclosures are the key solution to this problem.
See our video on Youtube
As a specialized supply-chain partner for medical device components, we not only provide cost-effective shielding solutions but also help clients understand:
✅ Why shielding is essential for medical devices
✅ How to choose the right shielding solution
✅ How to optimize costs without compromising performance
1. Why Must Medical Devices Use Shielding Enclosures?
(1) The Hazards of Electromagnetic Interference (EMI)
Medical devices operate in complex electromagnetic environments with interference sources such as:
Internal sources: High-frequency circuits, switch-mode power supplies, motors
External sources: Wi-Fi, 5G, crosstalk between medical equipment
Human body: Electrostatic discharge (ESD), muscle electrical signals
Real-World Case Studies:
ECG devices: 50Hz/60Hz power-line interference may cause baseline drift, affecting ST-segment analysis
MRI systems: RF noise reduces image signal-to-noise ratio (SNR), impacting diagnostic accuracy
Surgical robots: EMI may cause control signal delays, affecting operational precision
(2) The Core Function of Shielding Enclosures
A shielding enclosure acts as a Faraday cage, using conductive/magnetic materials to reflect or absorb interference, ensuring stable circuit operation.
2. How to Choose the Right Shielding Enclosure?
(1) Material Selection Based on Interference Type
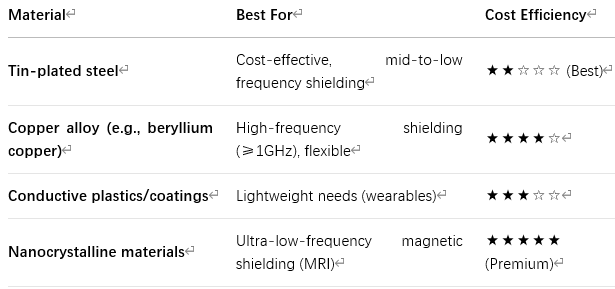
(2) Manufacturing Process Selection
Stamping: Best for high-volume, standardized production (lowest cost)
Laser cutting + bending: For complex geometries (±0.1mm precision)
3D metal printing: Prototyping or ultra-low-volume production
(3) Cost Optimization Strategies
Early design integration: Optimize shielding requirements during PCB layout to avoid costly revisions
Material substitution: Use tin-plated steel instead of pure copper where feasible
Standardized modules: Reduce tooling costs for small-to-medium batches
3. How to Predict Shielding Needs During Product Design?
(1) Key EMC Design Considerations
PCB Layout Phase:
Keep high-speed traces away from sensitive analog circuits (e.g., ECG amplifiers)
Reserve space for shield mounting (avoid mechanical conflicts)
Mechanical Design Phase:
Ensure low-impedance grounding (<0.1Ω contact resistance)
Use honeycomb vent patterns to prevent EMI leakage
(2) Testing & Validation Recommendations
Pre-compliance testing: Use near-field probes to identify interference sources during R&D
Certification acceleration: Choose shielding solutions pre-verified for IEC 60601-1-2
4. Our Value Proposition
As a specialized supply-chain partner for medical shielding solutions, we offer:
🔹 Free EMC consultation: Optimize designs for compliance and cost
🔹 Global material database: Multi shielding materials for all budgets
🔹 Rapid prototyping: First samples in 7 days, low-MOQ support
How to Start Cooperation? – A Simplified 3-Step Process
Requirement Alignment
Provide drawings or samples, specifying application scenarios (e.g., device type, interference sources, certification requirements).
Solution Optimization
Our engineering team evaluates materials, processes, and costs, providing feedback within 24 hours.
Delivery & Execution
Sign an NDA (Non-Disclosure Agreement) and proceed with production and quality inspection as scheduled.
Why is the Trading Model More Suitable for Medical Device Manufacturers?
✅ No Middleman Markups
Direct factory supply eliminates brand premiums.
✅ Resource Integration Capabilities
Match with the most suitable suppliers, free from single-factory technical limitations.
✅ Controlled Risks
Strict factory audits and batch inspections ensure every delivery meets standards.
We Are Not Just a Supplier – We Are Your Long-Term Partner in Medical EMI Shielding Solutions.



_TMrzP8.jpg)




